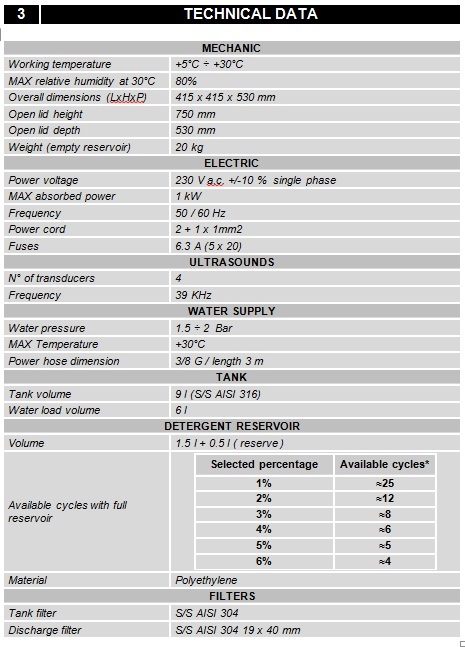
.jpg)

Risk factor
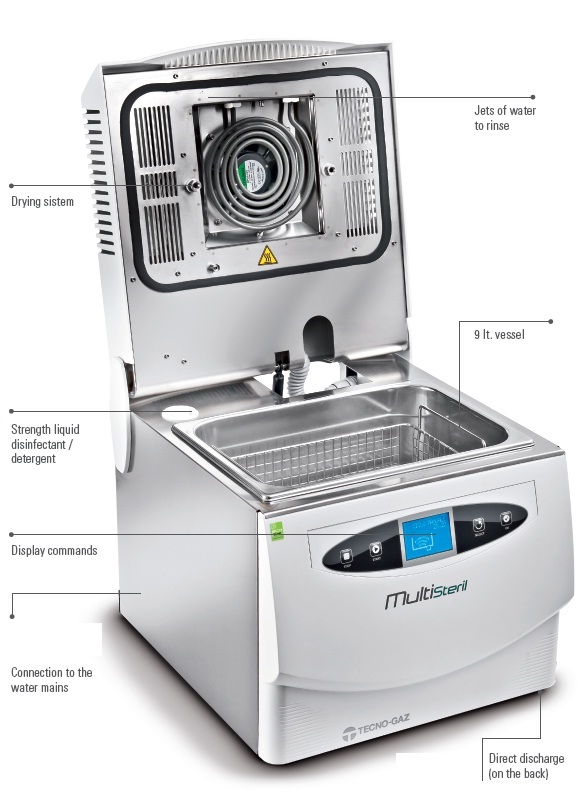
The manipulation of instruments and/or potentially infectious materials is a very important factor and must be strictly controlled, both for operators’ protection and the person(s) legally responsible for the surgery.
Multisteril eliminates any risk.
Management protocol
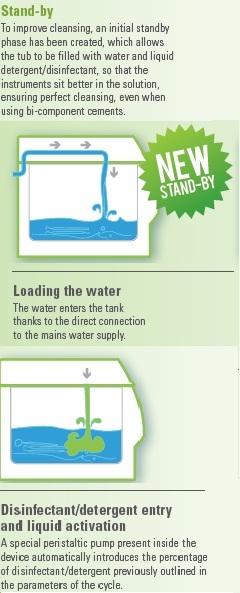
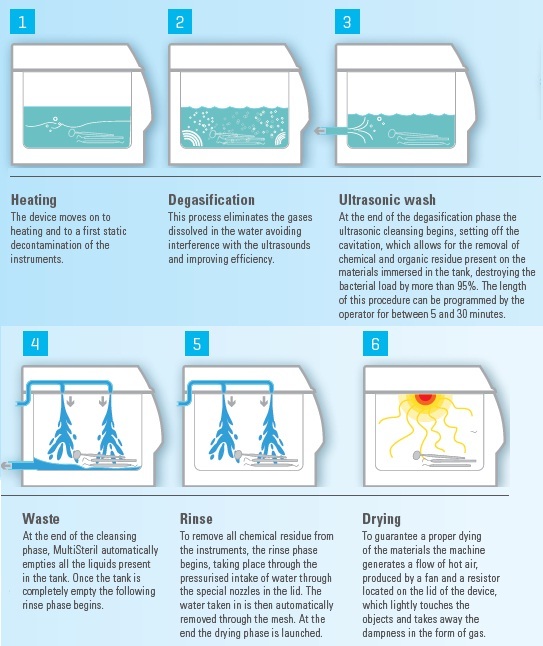
Sterilisation is ensured only if there is a correct procedure of material preparation.
Multisteril performs all the cycles correctly.
Spaces
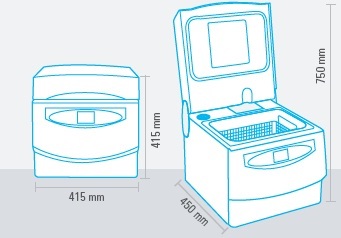
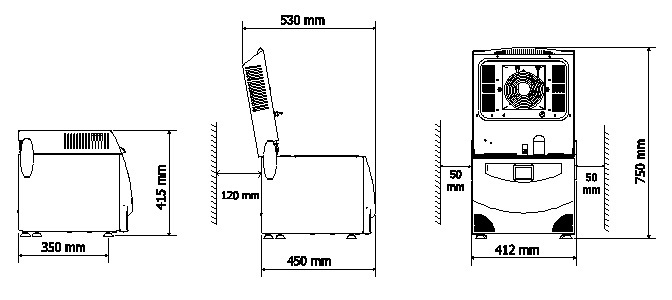
Space is another important problem. In fact, sterilisation rooms are often small; so, space rationalisation is very valuable.
Multisteril does everything in a 40 cm space.
Total costs
Cost is an important factor which must be kept under control.
With Multisteril, every cycle costs about 2 Euros.